Intermolecular and Interparticle Forces
Intramolecular forces are within a single molecule
Intermolecular forces are between two separate molecules
Intermolecular forces occur between two different molecules. For example, in water, the H molecule carries a slight positive charge, while the O molecule carries a slight negative charge. This results in hydrogen in one water molecule being attracted to the oxygen of another.
Intermolecular forces are Coulombic, like covalent and ionic bonds, but are generally weaker than intramolecular forces.
Important: Intermolecular forces help to explain surface tension of liquids, in addition to melting and boiling points. This is because the stronger intermolecular forces are, the more energy is required to disperse molecules, which increases surface tension or melting/boiling points.
- London Dispersion Forces (LDFs), are caused by very slight polarization of the nonpolar molecules due to changes in the electron field around them. London Dispersion Forces are the primary type of bonding among nonpolar molecules, but may also happen with polar molecules. The larger the electron field, the more polarizable it will be. This is typically the weakest type of intermolecular force but could be the strongest if the molecules are large.
- In Dipole-Induced Dipole bonds, a dipole will temporarily cause a nonpolar molecule to become polar in an attractive force. When the molecules move apart again, the nonpolar molecule will become nonpolar again. Ex: O₂ in water, H₂O experiences temporary polarization from the negative or positive dipoles of water.
- Dipole-Dipole is a type of intermolecular bond that is between any polar molecules. The molecules will generally orient themselves to maximize attraction and avoid repulsion. The strength of the reaction will be determined by the magnitude of the dipole.
-
Hydrogen Bonding is an extremely strong form of dipole-dipole bonding:
- Hydrogen only occurs between H and an O F or N atom
- Hydrogen bonding occurs between molecules: it is not truly “bonding”
- The Hydrogen bonded to an O F or N atom donates its electron, leaving just a positive nucleus. This will form an extremely strong attraction to other negative ions in a different molecule
-
When Ionic Compounds dissolve in an aqueous solution, they form Ion-Dipole Interactions:
- Melting and boiling points, surface tension, viscosity, and heat of vaporization will increase as IMF increases
- Vapor pressure, volatility will decrease as IMF increases
When two molecules have approximately the same number of electrons (same mass), then Hydrogen Bonding > Dipole-Dipole > LDF. If two molecules have vastly different amounts of electrons and the same types of IMFs, then the larger molecules will have stronger IMFs
Check Your Understanding:
Try this quick quiz to reinforce what you just learned about intermolecular and interparticle forces.
When comparing the intermolecular forces of NaCl and Octane (C₈H₁₈), which has stronger overall intermolecular forces and why?
Hydrogen bonding can occur between a Hydrogen and all of the following atoms except:
Which answer choice correctly ranks the intermolecular forces present in Dichloromethane (CH₂Cl₂) from least to greatest based on their strength?
When comparing CO₂ to HF, which of the following statements is true?
Properties of Solids
Solids have very strong interactions between particles:
-
-
Definite shape, volume, regular, crystalline structure, and only a vibrational degree of freedom
Types of Solids:
-
Ionic
- Alternating cation and anion in a crystalline lattice structure
- Generally high melting points due to strong attractions
- Brittle because of the repulsive forces if the solid is pushed
- Poor conductors of electricity when solid because electrons lack mobility. Good conductors as liquids or in aqueous solutions because electrons are more mobile
-
Molecular
- Formed exclusively by individual non-metal neutral molecules which form molecular lattice structures
- Relatively low melting and boiling points because of the relatively weak intermolecular forces
- Poor conductors of electricity at all states because electrons are held in covalent bonds
-
Metallic
- Formed by metallic elements exhibiting metallic bonding
- Good conductors of heat and good conductors of electricity because of their delocalized sea of electrons
- Malleable and ductile because the sea of electrons minimizes repulsion when bent/pushed
- Melting points vary, depend on metal
-
Covalent Network - Diamond
- Formed by distinct atoms bonded together in 3D structure
- Formed from carbon or metalloids like silicon, germanium, boron, etc.
- High melting point and very hard because of the strong attractions
- Poor conductors of electricity because the electrons are tightly held in covalent bonds
You should be able to identify these types of solids from a chemical formula alone.
Check Your Understanding:
Try this quick quiz to reinforce what you just learned about properties of solids.
What type of solid is characterized by relatively low melting and boiling points and poor electrical conductivity?
Silicon Dioxide (SiO₂) is most likely what form of solid?
Magnesium Oxide (MgO) is most likely what form of solid?
Solids, Liquids, Gasses
Understand the differences between solids, liquids, and gasses, the three main phases in chemistry
For H20 (water):
- Solid (Ice) is formed below 0 celsius, and has only a vibrational degree of freedom. Solid ice is actually slightly less dense than liquid molecule because of the crystalline structure and spacing
- Liquid water is formed from 0 to 100 celsius, has vibrational and translational freedom and faster moving particles. Liquid water is very dense because of the strong hydrogen bonding between molecules
- Gaseous water is formed above 100 celsius, and there is complete freedom and little to no attraction between molecules. Vibrating, translating, rotating freedom. Very low density and high intermolecular space
In general, solids have vibrational freedom (molecules can only vibrate in place), liquids have vibrational and translational freedom (molecules can move around each other but are still attracted), and gasses have vibrational, translational, and rotational freedom (molecules are free to move in all directions with little to no attraction)
Check Your Understanding:
Try this quick quiz to reinforce what you just learned about solids, liquids, and gasses.
What phase will generally allow molecules to both vibrate in place and move around one another, but not have complete freedom?
What choice gives the correct melting and boiling points (in degrees Celcius) for water (H₂O)
Ideal Gas Law
Ideal Gas Law: P V = n R T
Where
P: The pressure or force exerted on the interior surface of container walls by gas particle collisions (atm)
V: Volume (L)
n: Number of moles of the gas
R: Ideal Gas Law Constant, R = 0.08206 atm·L/mol·K
T: Temperature measured in Kelvin (K)
Using the Ideal Gas Law:
- P and V are inversely related
- P and n are directly related
- P and T are directly related
- R connects all of the units/values together
- The Ideal Gas Law assumes all gas particles to be of uniform size and weight (thus all having the same effect on the pressure of the system) regardless of chemical identity
Dalton’s Law: PTotal>= PA + PB + PC …
Dalton’s Law states that the total pressure of a sample of gas will be the sum of the pressure of each individual gas added up
Mole Fraction: Xi = ni / ntotal = Pi / Ptotal
Mole Fraction (χᵢ) is the ratio of the moles of one gas in a mixture to the total number of moles of gasses. Since Pressure (P) and moles (n) are directly related, the equation applies to the ratio of the pressure of one gas to the pressure of all the gasses in the mixture as well.
Check Your Understanding:
Try this quick quiz to reinforce what you just learned about the ideal gas law.
A sample of Neon gas is collected and places in a rigid container with a volume of 3.00 L. The temperature inside the container is maintained at 298 K. If the sample contains 0.500 mol of Neon, what is the pressure of the gas in the container?
A tightly sealed container with a volume of 2.25 L holds a mixture of oxygen and nitrogen gases at 298 K. If the partial pressure of oxygen is 0.80 atm and the partial pressure of nitrogen is 1.20 atm, what is the total pressure inside of the container?
A 5.0 L container is filled with carbon dioxide gas (CO₂). The temperature inside the container is 310 K. If the pressure inside the container is 2.0 atm, what is the mass of CO₂ inside the container, in grams?
In a 15.5 L container, some nitrogen gas (N₂) at 1.2 atm and some O₂ gas at 2.7 atm are stored. What is the number of moles of O₂ in the container if there are 13.6 total moles of the two gasses combined in the container?
Kinetic Molecular Theory
Kinetic Molecular Theory: Particles in gasses are in constant random motion, and between collisions will have a constant velocity and direction. After collisions the particles will have a new velocity and direction - collisions do not stick, they are elastic collisions. This summarizes the behavior of ideal gasses.
Note that Kinetic Energy is represented by the following equation:
KE = ½ m v²
At the same temperature, heavier particles will move slower than the lighter particles because of their higher mass.
Pressure is the result of molecules colliding with the container walls. Particles with different masses will exert the same pressure because they are moving at different speeds when colliding with the walls. Theoretically, higher molecular size would give the particle a larger volume and therefore less spacing (higher pressure), but this is generally assumed to be negligible, and ideal gasses will be assumed to have zero volume.
Check Your Understanding:
Try this quick quiz to reinforce what you just learned about kinetic molecular theory.
How does the temperature of a gas affect the average kinetic energy of its particles?
A particle has a mass of 2.0 x 10⁻²⁶ kg. What is its kinetic energy if it is moving at a speed of 600 m/s?
Deviation from Ideal Gas Law
In an ideal gas:
- Collisions are perfectly elastic - no sticking
- There is no attraction or repulsion between particles
- Particles have a negligible volume - they are just points
If all of this is true, the Ideal Gas Law is functional and can be accurately used.
However, in real gasses, there will be some volume and there will be intermolecular forces (IMFs). As IMFs increase, the actual pressure will decrease because the particles are more attracted to each other, reducing impacts on the container walls. Only at high temperatures do these IMFs become negligible because they are moving so fast.
Likewise, if molecular volume is increased (or volume of container is decreased), the pressure will also increase because there will be less space between the wall and between molecules. At lower pressures, this is close to negligible because there is more ample room between particles
Thus, gasses might exhibit non-ideal behavior when:
- Temperature is low (Increased IMFs)
- Pressure is high (Molecular volume might not be negligible)
- Particles have strong IMFs
- Particles have significantly large molecular size
Check Your Understanding:
Try this quick quiz to reinforce what you just learned about deviation from the ideal gas law.
All of the following conditions can cause gases to deviate from ideal behavior except:
A scuba diver fills his tank with compressed air (composed of nitrogen and oxygen). While diving at low depths, the pressure in the tank becomes high and the temperature drops significantly. What might cause a deviation from ideal gas behavior?
Solutions and Mixtures
A solution, or homogenous mixture, is a physical combination of varying states of matter that do not have varying properties based on location in the mixture or by molecule. A heterogeneous mixture does have differing properties throughout the mixture
Molarity may be used to express solution composition:
Molarity (M) = Moles of Solute / Liters of Solution
The solvent does the dissolving and is typically in a larger quantity. The solute is the substance being dissolved. Ex. Water is solvent and salt is solute in salt water.
Check Your Understanding:
Try this quick quiz to reinforce what you just learned about solutions and mixtures.
A student stirs some sand and salts into water until it is thoroughly mixed. The mixture is then left alone for an hour, and when the student comes back they notice the majority of the sand and salts have settled on the bottom of the beaker. Based on this observation, which statement is most accurate?
To gargle salt water, a woman dissolves 0.75 mol of NaCl in 250 ml of water. What is the molarity of the solution?
Representations of Solutions
Using particulate models to represent mixtures:
- Ion sizes should be correct in relation to one another
- Orientation of solvent and solute particles
- Concentrations of components in the mixture should be represented
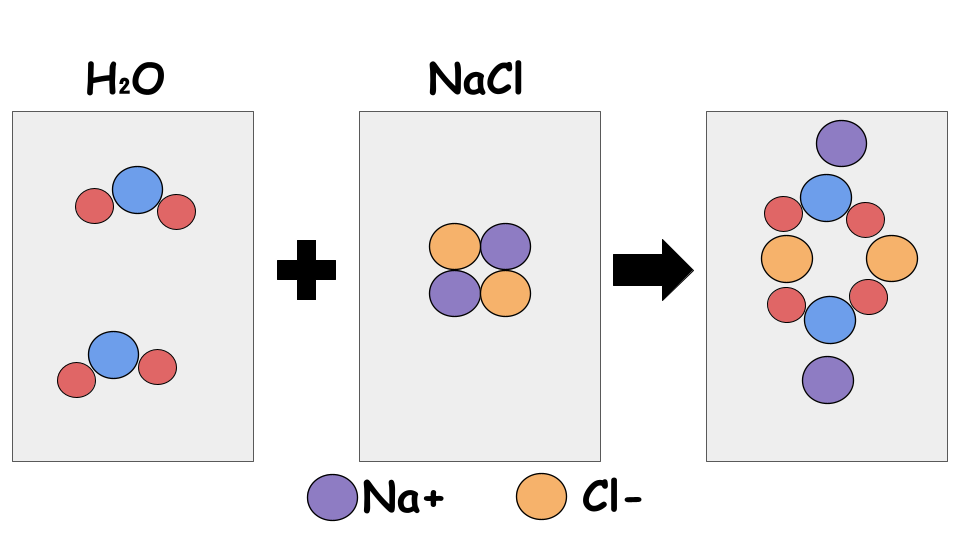
Notice the arrangement of particles so that positives charges are attracted to negative charges. Hydrogen is smaller in comparison to O, Na, and Cl.
Check Your Understanding:
Try this quick quiz to reinforce what you just learned about representations of solutions.
Which of the following best describes a visual difference you are likely to see between the particulate diagram of a solid and a gas?
Separation of Solutions and Mixtures
Filtration uses molecular size to filter, and this does not work with liquid solutions where particles are always closer in size and very small. It must also consider whether particles have significant intermolecular attractions.
Chromatography uses a thin paper to separate components of a solution using the solutions attractive/intermolecular forces:
- The chromatography paper is dipped into the solvent but the ink/sample spot is not submerged
- The paper is the stationary phase (it is not moving) while the solvent is the mobile phase (because it moves up the paper)
- If the stationary phase is highly polar, more polar components of a solvent will be more attracted to the paper (and will not travel very far), while the less polar components will have less attraction to the paper and will travel higher up the paper with the solvent
- If the stationary phase is nonpolar/not highly polar, more polar components of a solvent will be more attracted to the solvent rather than the paper, and will consequently travel higher on the paper. Less polar components of the solvent will have slightly less attraction to the solvent and will thus not travel as high on a low/no polarity stationary phase.
Distillation separates chemical species using the effects of intermolecular forces on vapor pressures:
- Because one component (liquid) may have a different boiling point when heating a solution, the lower boiling point will occur first, evaporating that liquid and sending it through a condensation tube to yield a pure liquid
- Liquids with weaker intermolecular forces will have lower boiling points and will be produced (the distillate) by distillation
Check Your Understanding:
Try this quick quiz to reinforce what you just learned about seperation of solutions and mixtures.
What mixture would be best separated by the filtration technique?
What separation technique would be most effective at separating ethanol (C₂H₅OH) and water (H₂O) given they have similar polarities and ethanol has a boiling point of 78.37°C?
Chromatography would be most effective in seperating which of the following mixtures?
Solubility
Substances with similar intermolecular properties will tend to be soluble (or miscible) in one another
- Ionic compounds dissolve in polar solvents (like water) because their anions and cations are strongly attracted to the positive/negative poles
- Molecular compounds without dipoles (only primarily London dispersion forces) will tend to dissolve in nonpolar solvents. This happens the strongest when there is higher magnitude of LDFs
- Polar mixed with a nonpolar substance will not mix, for example, water and oil will form a suspension
*"Like dissolves like" — the more alike two substances are in their intermolecular forces, the more likely they are to dissolve together.
Check Your Understanding:
Try this quick quiz to reinforce what you just learned about solubility.
All of the following compounds will be soluble in water except:
A manufacturer is designing a new water-based cleaning solution. Grease is generally made of long-chain hydrocarbons and does not mix effectively with water. To ensure the product dissolves grease effectively, which type of compound should they include in the cleaning solution?
Spectroscopy and the Electromagnetic Spectrum
Spectroscopy is the study of matter's interactions with electromagnetic radiation.
For AP Chem know the following types of radiation:
- Microwave radiation causes changes to molecular rotation levels
- Infrared radiation causes changes to molecular vibration levels (infrared has higher energy per photon, or a higher frequency and shorter wavelength, because vibrational states require more energy than molecular rotation)
- Visible and Ultraviolet radiation cause changes in electronic energy levels - this could cause electrons to jump to higher energy leves (highest frequency and shortest wavelength)
Check Your Understanding:
Try this quick quiz to reinforce what you just learned about spectroscopy and the electromagnetic spectrum.
What type of electromagnetic radiation can cause electrons to jump to higher energy levels?
Properties of Photons
Matter may absorb electromagnetic radiation and transition from its ground state to excited state.
Matter may also release electromagnetic radiation at its excited state, and transition back to the ground state.
A spectrometer detects how a sample responds to electromagnetic radiation, processes the resulting spectrum, and produces a data readout.
The wavelength and frequency of a photon are related by the following equation:
c = λ v
Where:
c = the speed of light, 3 X 108 m/s or 3 X 1017 nm/s
λ = the wavelength, in m or nm
v = frequency, in Hz (Hertz) or s⁻¹
When a photon is absorbed or emitted from a molecule, the molecule will lose/gain the amount of energy equal to the photon. Energy is represented in Planck’s Equation:
E = h v
Where:
E = Energy of the photon (J)
h = Planck’s constant, 6.626 X 10⁻³⁴ J/s
v = frequency, Hz (Hertz) or s⁻¹
Note that you do not need to memorize these formulas, the constants, or have a deep understanding of why they work, but you should be able to apply them in context, plugging in given values to find the missing variable. The formulas and constants are on the AP chem formula sheet you will be given during the AP Chem Exam.
Check Your Understanding:
Try this quick quiz to reinforce what you just learned about properties of photons.
A spectrometer detects a photon with a wavelength of 250 nm. What is the energy of this photon in joules?
A photon has an energy of 3.31 × 10⁻¹⁹ J. The wavelength of this photon, in nanometers, is closest to which of the following choices?
Beer-Lambert Law
Instruments like spectrophotometers and colorimeters can be used to determine the absorbance of a chemical species
The Beer-Lambert Law relates the absorption of light by a solution:
A = ε b c
Where:
A = Absorbance Measurement
ε = Molar Absorptivity (how intensely a sample absorbs light of a specific wavelength)
b = Path Length
c = concentration
Usually the path length and the wavelength will be constant to measure the absorptivity at different concentrations
Again note that this formula will be given on the AP exam, so you should focus on understanding how to plug any given values in to use it to find an unknown.
Check Your Understanding:
Try this quick quiz to reinforce what you just learned about the Beer-Lambert Law.
A spectrophotometer measures the absorbance of a solution to be 0.75. If the path length is 1.00 cm and the molar absorptivity (ε) is 1500 M⁻¹·cm⁻¹, what is the concentration of the solution in mol/L?