Introduction for Reactions
When a substance undergoes a physical change, the composition of the substance does not change:
- Physical properties like shape, or solubility at different temperatures may change
- Phase changes and separation of mixtures are examples of a physical change
When a substance undergoes a chemical change, the composition of the substance does change during a reaction:
- Chemical properties like flammability, or reactivity with acids may change in the new composition
- Production of heat, light, new colors, or a precipitate (new substance formed) often signifies a chemical reaction taking place
Check Your Understanding:
Try this quick quiz to reinforce what you just learned about introduction to reactions.
Which of the following is most likely a chemical change?
A chemist places a small piece of solid Iodine into a sealed glass container and warms it. After several minutes, the iodine has completely disappeared from the bottom of the glass container. Which of the following best describes the change that occurred?
Net Ionic Equations
In all physical and chemical changes, the same number of elements on the products side must be on the reactants side to comply with Law of Conservation of Matter
Balanced molecular equations show all species participating in a reaction. Ionic equations show ions in an aqueous solution to easily identify spectator ions (any species not actively undergoing change) - Net Ionic Equations do not include the spectator ions, and only represent the ions undergoing change
- To write a net ionic equation, first write the balanced molecular equation
- Next, break all aqueous compounds into their respective ions to form the complete ionic equation
- Finally, cancel out all spectator ions (ions that appear unchanged on both sides of the equation) to form the net ionic equation
More detailed examples coming soon...
Check Your Understanding:
Try this quick quiz to reinforce what you just learned about net ionic equations.
When aqueous solutions of silver nitrate (AgNO₃) and sodium chloride (NaCl) are mixed, a white precipitate forms. What is the correct net ionic equation for this reaction?
A student mixes solutions of lead(II) nitrate (Pb(NO₃)₂) and potassium iodide (KI). A bright yellow solid forms. What is the correct net ionic equation for this reaction?
Representations of Reactions
Chemical Reactions may be represented in various ways:
- Chemical symbols may be used to represent reactants, products, and states of matter
- Ex: N₂(g) + 3H₂(g) → 2NH₃(g)
- A particulate diagram may be used to represent a reaction (see below)
- Note that in a particulate diagram the excess reactant may be displayed with the products depending on the amount of the reactants initially present
- Sizes, charges, and orientations may be displayed in a particulate diagram
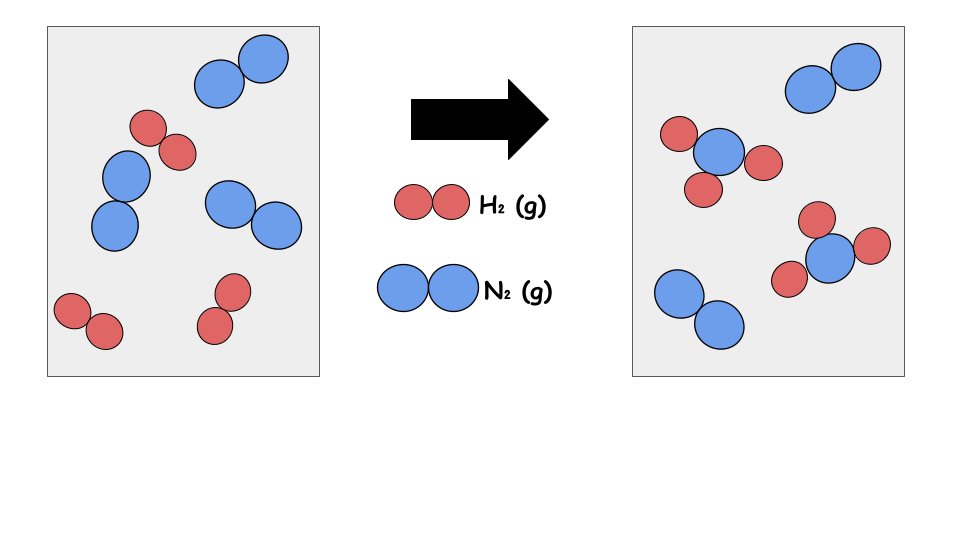
Notice there are the same amount of each element on either side.
Check Your Understanding:
Try this quick quiz to reinforce what you just learned about representations of reactions.
In balanced particulate diagrams and chemical equations, what is always true?
Physical and Chemical Changes
Physical Process:
- Involves changes in intermolecular forces
- Bonds are generally not broken or formed
- The chemical composition does not change
Chemical Process: Chemical changes will have breaking or forming of bonds and a changing of chemical compounds. Ex: A nail rusting, or bread baking
* Remember that Hydrogen Bonds are not bonds, but forces of attraction, thus the breaking of hydrogen bonds does not signify a chemical change
Note that dissolution can be argued as a chemical or physical change. For example, salt (NaCl) can have its bonds broken when dissolved in water, and the Na cation and Cl anion will become surrounded by H2O molecules.
Check Your Understanding:
Try this quick quiz to reinforce what you just learned about physical and chemical changes.
Which of the following scenarios most likely represents a physical process rather than a chemical change?
Stoichiometry
A balanced chemical equation may be interpreted as a ratio of moles. This allows us to determine the limiting reactant, excess reactant, and theoretical yield
Note that the volume of any gas at STP is 22.4 L
Check Unit 1.1 (Moles and Molar Masses) first if you need a refresher on mole conversions.
Ex. Given the equation 2Mg(s) + O₂(g) → 2MgO(s). If 12.2g of magnesium (Mg) react with excess oxygen, how many grams of MgO are produced?
| 12.2 g Mg | 1 mol Mg | 1 mol MgO | 40.3 g MgO |
| 1 | 24.31 g Mg | 1 mol Mg | 1 mol MgO |
Multiply the top values and divide by the bottom values to get the final answer. 12.2 g Mg x (1 mol Mg / 24.31 g Mg) x (1 mol MgO / 1 mol Mg) x (40.3 g MgO / 1 mol MgO) = 20.2 g MgO
Notice how all the units cancel out by having one on top and one on the bottom. This leaves only the ending units, g of MgO, leftover.
Check Your Understanding:
Try this quick quiz to reinforce what you just learned about stoichiometry.
Given the equation 2HCl + Na₂CO₃ → 2NaCl + H₂O + CO₂, how many moles of CO₂ will be produced if 3.7 mol of HCl is mixed with 1.0 mol of Na₂CO₃?
Given the balanced equation 2Al(s) + 3Cl₂(g) → 2AlCl₃(s), if a chemist reacts 10.8 grams of aluminum (Al) with excess chlorine gas, how many grams of AlCl₃ will be produced?
Introduction to Titration
Titration is an experiment where a solution of known concentration (the titrant) is added through a burette to a beaker with a solution of unknown concentration (analyte) to determine the number of moles in the unknown solution.
The equivalence point is reached when the titrant from the burette has completely reacted with the analyte. An indicator is added to the analyte so it will change colors at the equivalence point. The end point is the point where the indicator changes color.
A titration curve may depict progress of titration. The point on the curve where the pH change is greatest represents the volume of titrant needed to react with the analyte in stoichiometric ratios.
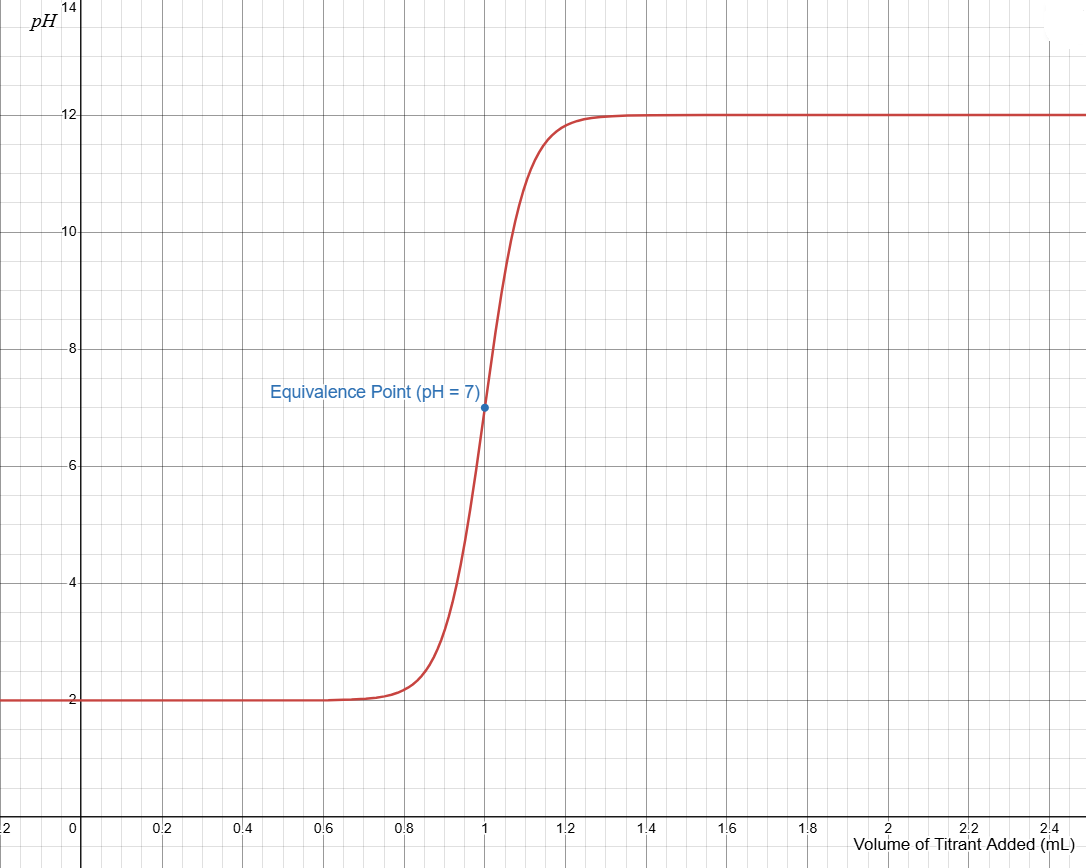
Example titration curve shown above: this is a strong acid titrated with a strong base. The equivalence point is where there are equal moles of acid and base, this is at the steepest point in the graph.
Check Your Understanding:
Try this quick quiz to reinforce what you just learned about titration.
During a titration, if the colored indicator can be seen, what does this signify?
The equivalence point in a titration is the point at which
Types of Chemical Reactions
Types of Chemical Reactions:
-
Acid-Base Reactions
- In Acid-Base Reactions, neutralization reactions will occur
- Based on Bronsted-Lowry Acids and Bases, the acid will donate a proton (H+), and the base will accept a proton (H+)
- The acid forms a conjugate base (by removing the proton or H+) and the base forms a conjugate acid (by accepting the proton or H+)
- Ex: HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l). HCl is the acid and NaOH is the base, NaCl has lost a proton and is the conjugate base (specifically the Cl- ion), H₂O gained a proton and is the conjugate acid.
- Redox reactions involve the transfer of one or more electrons between reactants. Electrons are transferred from the substance that is oxidized to the species that is reduced
- Oxidation is loss of electron, reduction is gain of electron: Oil Rig
- Use oxidation numbers to determine reduction and oxidation species
- Ex: Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s). Zn is oxidized (loses electrons) and Cu²⁺ is reduced (gains electrons). You may not always have them charges in the equation so you need to be able to determine them
- Precipitation reactions involve mixing ions in aqueous solutions to produce an insoluble ionic compound - the precipitate
- All sodium, ammonium, nitrate, and potassium salts are soluble in water
- Ex: AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq). AgCl is the precipitate that forms because Ag⁺ and Cl⁻ form an insoluble compound
When determining what type of reaction is occuring, you should check for acid-base and redox reactions first because precipitation reactions can be more difficult to identify.
Check Your Understanding:
Try this quick quiz to reinforce what you just learned about types of chemical reactions.
The equation Pb(NO₃)₂(aq) + 2KI(aq) → PbI₂(s) + 2KNO₃(aq) represents what type of reaction?
Which substance is being reduced in the redox reaction shown: Fe(s) + 2HCl(aq) → FeCl₂(aq) + H₂(g)?
In the acid-base reaction NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq), which of the following is a correct Bronsted-Lowry acid-base conjugate pair in this reaction?
Introduction to Acid-Base Reactions
Remember Bronsted-Lowry Acid and Bases. The acid donates a proton (H⁺) and the conjugate base is what remains after the acid has donated its proton. The base accepts a proton and becomes a conjugate acid.
Note that some substances, such as water, may serve as either the acid or base in a reaction.
Check Your Understanding:
Try this quick quiz to reinforce what you just learned about acid-base reactions.
An acid-base reaction follows the formula HCOOH(aq) + H₂O(l) ⇌ H₃O⁺(aq) + HCOO⁻(aq). Which of the following is the correct Bronsted-Lowry acid and conjugate base pair in this reaction?
In acid-base reactions, H₂O acts as...
Oxidation-Reduction (Redox) Reactions
Remember in redox reactions, "OIL RIG" - Oxidation Is Loss (of electrons), Reduction Is Gain (of electrons).
Redox reactions can be separated into their half-reactions (the reduction and the oxidation reactions), then the two separate equations must be balanced to contain an equal number of electrons gained and lost. The two equations can then be re-combined to produce the net-ionic equations
Ex. Mg(s) + Al³⁺(aq) → Mg²⁺(aq) + Al(s)
First write half equations: Mg(s) → Mg²⁺(aq) + 2 e⁻ and Al³⁺(aq) + 3 e⁻ → Al(s)
The electrons are not balanced, so multiply both half reactions by the electron coefficient of the other to balance them
Balanced half-reactions: 3Mg(s) → 3Mg²⁺(aq) + 6e⁻ and 2Al³⁺(aq) + 6e⁻ → 2Al(s)
Now combine half reactions and cancel the electrons out since they appear on both sides:
3Mg(s) + 2Al³⁺(aq) → 3Mg²⁺(aq) + 2Al(s) is the final balanced net ionic equation.
Check Your Understanding:
Try this quick quiz to reinforce what you just learned about oxidation-reduction reactions.
In the redox equation Fe(s) + Cu²⁺(aq) → Fe²⁺(aq) + Cu(s), how many electrons are transferred?
Which answer choice gives the correct balanced oxidation and reduction half-reactions for the redox equation Cr₂O₇²⁻(aq) + Fe²⁺(aq) + H⁺(aq) → Cr³⁺(aq) + Fe³⁺(aq) + H₂O(l)?/p>